NCI director Ned Sharpless gave a talk titled “Working Together to End Cancer as We Know It: 50 Years of the National Cancer Act.” at the Calabresi Memorial Lecture at Yale Cancer Center Nov. 2, 2021. Read The Cancer Letter‘s coverage of the talk here.
The transcript of Sharpless’s Calabresi Memorial Lecture follows:
Sharpless: Thank you for that kind introduction. It’s good to see old friends, at least virtually. I wish I could be there in person. I love New Haven and we will have to take a rain check on this.
I’m really excited today to do this for two reasons—one, it’s an opportunity to talk about the National Cancer Act and how important that’s been on its 50th anniversary. But also, I think it’s an opportunity to recognize a real giant among cancer researchers and cancer caregivers and such an important leader in our field.
As has been said, the Calabresi family has a deep connection to Yale, including Paul’s father—who was a cardiologist, I’m told—and his mom had a degree from Yale, and we’ve heard about Judge Calabresi and his eminent work with Yale. And we hear about the next generation of Calabresis having these deep Yale connections.
Paul Calabresi also had a deep connection to the National Cancer Institute. I think his career actually began doing field work for the NCI, then he served the NCI in several capacities—including as chairman of some of our most important advisory boards, the National Cancer Advisory Board and the President’s Cancer Panel, as Vince alluded.
And the honorable Guido Calabresi, I’m told, is here today. Let me offer a heartfelt recognition to all the Calabresi family for what they’ve contributed to Yale, improving what I believe is the human condition through their work.
I’m also pleased that Dr. DeVita is here. Vince is a giant in our field and has a direct connection to what we’re talking about today, the National Cancer Act. Vince joined the NCI in 1963 and was NCI director from ‘80 to ‘88. Dr. DeVita has been a wealth of good advice to me in this role. I found his book, titled The Death of Cancer, a really interesting thing to read before I started as NCI director. I recall having a very interesting conversation with Vince about the FDA prior to me going to the FDA to be acting commissioner for seven months.
It was really informative to have that perspective in the back of my head as I worked regulating food and drugs. So, I think it’s a fitting memory that we’re going to talk about the National Cancer Act today, given Dr. Calabresi’s connections to it. His contributions to cancer research, cancer care, and the infrastructure, so to speak, of our research capabilities made him a real giant in our field.
I’m talking about his work in understanding the pharmacology of cancer chemotherapy, his work in combining chemotherapy with other modalities, his leading-edge research in geriatric medicine—which I think is very prescient for a cancer researcher—his devotion to patient care, which has really empowered his research activities, and his leadership on countless boards, committees, institutes, academies, societies, and various other governing bodies.
But I think perhaps most important is his invaluable membership to a real generation of leaders in cancer research and cancer care, including one of my old bosses, Dr. Bruce Chabner at the MGH. At the NCI, we honor Dr. Calabresi’s contributions with a specific grant in his honor. It’s the Paul Calabresi Career Development Award for Clinical Oncology.
These are K12 grants that are really designed to prepare oncologists for effective scientific careers, in particular by pairing them with basic scientists. I was actually the PI of the University of North Carolina’s Calabresi award many years ago, and I know how important an award that is. It’s fitting to honor Dr. Calabresi with a training award, for that stage of someone’s career, given his terrific legacy of mentorship and training.
So, today I would like to talk about the National Cancer Act and how that changed from the period of Dr. Calabresi’s career to modern day. I think the efforts of Paul along with other luminaries of the past five decades really drove and made possible the tremendous progress we’re seeing today in cancer care and cancer outcomes. Their work provided this progress in the past, but also made possible these opportunities that I believe lie before us, and that will shape the future of cancer research and cancer care.
I think it’s hard to overstate the importance of the National Cancer Act in 1971. For those of you who are younger and don’t know a lot about the NCA, it did not create the NCI. The National Cancer Institute dates back to the 1930’s.
But I would argue that in many ways, the National Cancer Act created the modern NCI. The thing that we recognize today as the National Cancer Institute united patients, doctors, scientists, industry, and government in a common vision.
Impact of the National Cancer Act
From my perspective, I think the NCA really did three important kinds of things. So, first off, we heard from Vince about how it provided additional funding for cancer research. I’m sure that that extra funding was very important to Dr. Carl Baker, who was director of the NCI at the time. I think more support for cancer is always important, but I would actually argue that the funding was probably the least important of the many important things the NCA did.
A second type of activity the National Cancer Act did was it gave the NCI a bunch of new authorities and created new critical infrastructure that led to some of the modern capabilities of the National Cancer Institute.
It encouraged the NCI to create a national database of cancer statistics, which led to the SEER program, arguably the most important set of cancer statistics in the world. It created Frederick National Lab, which is a way of doing research the NCI uses. It really invigorated and provided the framework for the modern cancer center program that we’ve heard about. It made the NCI director presidential appointee.
It did a bunch of other things like the President’s Cancer Panel and the National Cancer Advisory Board, things that were really important. I think those authorities and new infrastructure were a really important part of the NCA, but maybe the second most important thing it did.
The third thing that I believe the National Cancer Act did, and arguably the most important thing the National Cancer Act did, was it made cancer something that we could talk about as a society. It turned cancer from a disease that had stigma associated with it, a diagnosis that was sort of hidden in the shadows, and it brought it out into the light. It really spurred the modern interest we have in cancer, the ability to talk about cancer, the ability to work on cancer, and the modern cancer advocacy movement, which has been so important. So, it was a really important act and it did things of terrific significance.
But, as visionary as the National Cancer Act was, it was also naïve. Many of the individuals involved at that time thought we’d have a cure for cancer very quickly, in five to 10 years. I think this was motivated by the experience of antibiotics and sepsis in the early 20th century.
Obviously, things didn’t work out that way—cancer turned out to be a much more difficult problem than we understood in 1971. But, we have worked for five decades to better develop that basic science understanding of cancer, and today we have a much better understanding of the molecular underpinnings of cancer and that better understanding is paying huge dividends for patients.

There are lots of ways to look at the remarkable progress in cancer over the last few decades. Some of the various takes on that are shown here. I believe that we’ll look back on this period today as a golden age of cancer research, where we really began to take the basic science understanding of cancer and apply it to human benefit in a very direct way.
We’ll think about this era today the way we think about antibiotics and the early 20th century for infectious diseases. It doesn’t always feel that way, I know—I realize that the burden of cancer in American society is still very significant—but from my perspective, the progress in cancer is really remarkable.
Here are a few lines of evidence. First, on the far left here, we see this decline in cancer mortality. This started in the early 1990s. Cancer mortality peaked in the United States and has declined for both men and women since then, for lots of reasons—better cancer screening, tobacco control—lots of things have conspired together to lower cancer mortality rates in the United States. In recent years, this has picked up markedly. I think in recent years, some of those massive declines in cancer mortality are related to better therapy.
For example, I show statistics here for lung cancer, where a bunch of new therapies—kinase inhibitors, immune checkpoint inhibitors, better radiation and surgery, et cetera—have all led to a remarkable decline in cancer mortality on the order of 6%, from 2013 to 2016 per year. So, a fairly sharp decline in the most lethal cancer in humans.
This has been matched by a remarkable increase in FDA approvals of drugs and devices and other medicines for cancer patients—a remarkable period of productivity. I remember when I started as a fellow in this business, you could go a whole decade and not really have amazing new drugs approved in cancer care. Now it’s a monthly event at the FDA. There was a period in 2020 where I think we had seven lung cancer drugs approved in the same month—really paradigm-changing new therapies.
I think there’s real scientific excitement in cancer research. And that’s shown down at the bottom right here; that’s the graph of applications for National Cancers Institute funding. We can see this massive increase since 2013, a nearly 50% increase over about a seven year period in applications for funding from the NCI.
This is a mark that people have great new ideas for cancer therapy and are coming to our field with new proposals and new ways of treating cancer. That includes physicists and mathematicians and other kinds of biologists working with new clinical approaches, all seeking support from the National Cancer Institute for their research.
This also creates a problem—albeit, I would argue, a good problem—which is tremendous competition for funding for the NCI. Vince mentioned the 50% success rates for grant funding back in his era—it was as low as 8% earlier in my career at NCI.
We have now, through fairly Herculean measures, gotten it up to 11%, but that is still a very low success rate for grants at the NCI, and something we’re deeply concerned about, because that is the pool of grants where paradigm-changing ideas come from, the things that really move the field for patients. So, improving support for investigator-initiated science remains a top priority for the NCI.
I think many Americans have heard of these advances and really take them for granted. It’s like computing power, automobile mileage; we just sort of expect these things to get better indefinitely without realizing all the work that went into them. But, that was not the case in 1971, that wasn’t even the case in the early 1990’s, it’s really become a feature more recently. That is really built on the molecular understanding of cancer biology that we’ve developed in the past 50 years.
And now, I think we should talk about where we go from here, how we use this progress of the last five decades as a bridge to the future. This next period of bridge building will build on that momentum that we’ve established over the last 50 years. It’s not just the momentum of the fundamental understanding of cancer and this knowledge base, but the keen scientific insights of those on whose shoulders we stand now, people like Dr. Calabresi and Dr. DeVita.
Cancer Moonshot
For the next few minutes, I’d like to talk about how we’re going to build that bridge to the future, building on this progress. What motivated my talk’s title today is a quote that’s been made frequently by the president. President Biden has said many times now that he’d like to end cancer as we know it. And I think Paul Calabresi would be gratified to know that we have this president in the White House with an intimate connection to cancer research, who knows what our work means for the American public.
President Biden and the first lady have a very strong personal connection to cancer. The story of their son’s death to glioblastoma is well-known to all of us. They’re also firm believers in the power of cancer research. The tragedy that befell the Biden family led to then Vice President Biden’s leadership of the Cancer Moonshot six years ago. The current administration, as I said, is calling for all of us as a community to end cancer as we know it.
We think that this problem is much bigger than just the NCI. The NCI is obviously a part of this, but this would require all the powers of the federal government, as well as advocacy and caregivers outside of the federal government. In considering the achievements of the past 50 years and how to steer the future of cancer research, we’ve been thinking this through at the NCI. What does it really mean to end cancer as we know it? How do we know cancer today? What would it mean to change that experience of cancer? What would that take?
Redefining the end of cancer
First, let me be clear. There is no mention of eradicating all cancer. Based on what we know about human biology today, we don’t believe that’s possible at the NCI, at least any time soon. But, we do think we can dramatically change the experience of cancer—that is, the tragedy of cancer, the way the American public knows cancer today.
To get at this, we have to be upfront about the uncomfortable realities about cancer as we know it today. I mentioned a lot of the progress, and that progress is very exciting and has been very good, but we still have a long way to go.
In the United States, 600,000 Americans still die from cancer each year, cancer is still the leading cause of death for children from disease, and cancer costs the nation hundreds of billions of dollars every year in terms of treatment and lost productivity. Even when we’re able to cure patients with cancer, too often this comes at the cost of severe treatments with significant long-term toxicities. Cancer for many patients is still a very devastating and life-changing diagnosis.
For people with a new diagnosis of cancer, telling them about all this great progress the last few years, that’s really small comfort. They don’t really want to hear from the NCI director about the record number of grant applications or FDA approvals or new infrastructure. They like to see cures or at least better treatments for their cancer, which provides them more time.
I once treated a woman in her early forties for metastatic triple negative breast cancer. We tried the usual therapies and it wasn’t working. It wasn’t going well. We were discussing what therapy to try next for her. And I did what we train our junior oncologists to do—I asked her what her goals were for therapy. I said, what do you want to get out of this next round of treatment?
As I said, this is something we inculcate in our medical students, and we sort of beat this habit into the residents and fellows to ask the patient what they want from therapy. It’s an important thing to do, but in some ways it’s also kind of a dumb question, right? It’s no mystery what our patients want.
They generally want better treatments for their cancer. They want a cure for their cancer. They want their cancer to go away and never come back. So, the goals for therapy are usually pretty obvious. What we’re really doing in this period is trying to get them to understand what’s possible—managing expectations based on what we believe we can deliver.
This patient told me that she knew she would die of cancer. She knew she had untreatable, refractory metastatic disease, and she had no illusions of being cured, but she wanted more time. She had three children who were middle school aged at the time, and her goal of hers was to see them graduate from high school.
That’s all she wanted, just a few more years. It didn’t seem at the time like an unreasonable request, given all this progress and work we’ve had in cancer, but we couldn’t even do that for her. She died about a year later.
I’ve argued many times before that many of us in the cancer community have become afraid about talking about curing cancer. I believe I made this exact point at Yale in 2017, soon after I became NCI director. I know why using the word ‘cure’ around patients causes so many problems for caregivers.
I understand the worry about providing false hope and empty promises. I know that we have gotten into this habit of qualifying our language all day long, of caveats and disclaimers and talking about things like disease-free survival and remission and whatever metric’s in vogue that day.
But patients, I think we should be clear, still want to be cured of their disease. And if that’s not possible, they want their cancer to be turned into a manageable chronic disease so they’ll have more quality time with their loved ones. So, that’s really what we’re talking about when we say ending cancer as we know it, or knowing cancer today, and that’s what the president wants us to do. At the National Cancer Institute, we’ve been thinking a lot about what it means to know cancer.

One way to think about this is things that are true about cancer today—true statements that we would like to make untrue in some way. If we can make these things untrue, then in doing so, we would change cancer as we know it. So, I’ve spoken at length already about cancer mortality, in this box here in the lower left.
I gave a lecture last April at AACR when I described how I believe a strong reduction in cancer mortality is possible, building on momentum we’ve seen over the last 30 years. I talked about the things that we could do to try and cut cancer age-adjusted mortality in half, from its peak in 1990 to half of that in the next few years, and some approaches that one could take to try and get there as quickly as possible.
So, those are things that would really drive down age-adjusted mortality quickly. You can say this is really the ultimate measure of our progress in cancer, how many people are dying of cancer. But there is a lot more to the experience of cancer than just mortality.
Today I wanted to focus on some of the other topics that we talk less about. A few are shown here. For example, we have too few ways to prevent cancer. Many treatments are so toxic that they are intolerable and cause lifelong morbidity. Too many patients are stymied by the complicated logistics of cancer care, creating these disparities because of access to care. I know we can all think of other statements that are true about cancer and things that we’d like to make untrue about cancer.
I believe it’s within our power to deliver on the president’s call to action, to confront the current reality of cancer and unravel it, to take today’s sad reality, and realize a better future. In the months ahead, I want all of us in the cancer community to consider the steps we can take to solve these problems as we’ve solved many other related problems for the past five decades.
I don’t really have time today to delve into all of these, so I thought I’d pick a few to talk about. The ones that I boxed here are the areas where I’d like to focus. We’ve already talked about mortality a bit, so I thought I’d take on early detection and screening, health inequities, and refractory and rare cancers.
Screening and detection
In 1971, cancer screening and detection was really in its infancy, but we now know that screening and early detection are really powerful tools for cancer outcomes in individuals, but also at the population level. It’s clear that development of effective screening approaches has been transformative, but we think things are really early in this field still, and believe screening and detection could be even more impactful than they are today.
Now we have effective screening tools for cervical cancer, breast cancer, colorectal cancer, and lung cancer. And even though their uptake is not as good as we would like, especially for lung cancer, the screening modalities for these diseases have had a dramatic impact on U.S. cancer mortality already.
I spoke with someone recently who had been diagnosed with early stage breast cancer, with screen-detected breast cancer found in a mammography. She described to me what an inconvenience this was, how it had been a little frightening at first, but then it just had become more of a hassle.
She’d had a minimal surgery and a brief course of radiotherapy, and was told that she would enjoy an excellent prognosis. That’s really the kind of experience we want to see for more types of cancer. I mean, can you imagine anyone in 1971 talking about a diagnosis like that being an inconvenience? That’s a problem that is in some ways a good problem.
But, even after many advances in detecting and treating cancer, the uncomfortable reality is that we still lack effective ways to detect many types of cancers before they spread and become more difficult to treat. The cancer types with some of the worst outcomes, frankly, are those where the disease can only be detected typically when it’s too late to treat effectively—pancreatic cancer and glioblastoma, et cetera.
Lung cancer is an area where the National Cancer Institute’s work should be highlighted. It’s had an important impact on early cancer screening and early detection. I think this group will be aware of the National Lung Cancer Screening Trial, which was a landmark study led by the NCI that showed that CT scanning could reduce mortality from lung cancer in specific populations related to age and history of smoking.

This result was confirmed by a similar European trial, and now low-dose CT screening is really considered the standard of care for patients of a certain age with certain histories of tobacco use as an effective means of reducing lung cancer mortality. This is an example of how we can rigorously test that approach and move it into broad community practice, and then refine it further through study.
This is also an important illustration of some very critical nuances related to cancer screening. For example, the screening guidelines that were finally established in 2013 by the United States Preventative Services Task Force, the USPSTF, excluded large numbers of patients from screening because of the cutoffs that were chosen.
This particularly applied to women and African American individuals who had lower smoking histories, not as many pack years. These individuals hadn’t smoked enough to meet the cutoffs, but they nonetheless face a higher risk of dying from lung cancer. The NCI sought to address this issue by performing modeling in our CISNET network.
We concluded that screening guidelines should be amended to protect patients with a more modest history of tobacco use. Based on that work, the latest revision of the USPSTF guidelines for lung cancer lowered those thresholds, a change that is a particular benefit to female and African American smokers who are now eligible for screening. A side note, by the way—a similar recent USPSTF change was made to colonoscopy and colorectal screening guidelines, also based on NCI-sponsored CISNET modeling.
The main problem with lung cancer today is—this is vastly underutilized for reasons that I do not completely understand. We’ve modeled what a more robust uptake of lung cancer screening could mean in terms of overall cancer mortality in the United States. It’s a real opportunity, and the NCI is funding many studies in this field of dissemination and implementation science to understand why an effective screening modality is so vastly underutilized.
The story of lung cancer screening shows how the NCI can play a really important role in developing the preliminary science, disseminating that, and then refining these recommendations all for public health benefit. Broader adoption of proven methodologies like lung cancer screening will be important, but there are also exciting new technologies for early cancer detection. One particular approach is the so-called multi-cancer early detection tests, or MCEDS.

The idea here is a single test, usually a blood test, done on otherwise healthy individuals at some regular interval yearly, to diagnose several cancers at once by detecting features of the cancer in a single analyte—the tube of blood. There are many approaches to this—there’s DNA methylation, there’s cell-free DNA, there’s exosomes, etc.
I believe this concept holds great promise, and these technologies are evolving rapidly and entering large scale clinical testing as we speak. These approaches could potentially reduce cancer mortality at the population level, but they have to be rigorously evaluated in a timely manner.
ARPA-H
As I think this group is aware, cancer screening is a tricky business because there’s always this worry about over-diagnosis and over-treatment and the ability to harm patients through cancer screening. Evaluating these technologies will be challenging. Parenthetically, for those of you who have been following news in DC, you will have heard about this new entity called ARPA-H, which at this point is still a proposal being taken up by Congress to create a new agency akin to DARPA. DARPA is the Defense Advanced Research Projects Agency, So ARPA-H would be the Advanced Research Projects Agency for Health.
This would be within the NCI, but with different structures and authorities to enable the rapid development of high-risk, high-reward projects. I believe, and others have also stated that, ARPA-H might be a good instrument for evaluating a new technology like this, as there’s this very pressing need to evaluate these technologies as soon as possible.
Health disparities and workforce diversity
Let me turn to another major problem that we have failed to adequately address, and that is cancer health disparities, and inequity in cancer care. This is a whole constellation of issues that drive disparities and outcomes for our patients. We face important disparities in cancer diagnosis, in treatment, trial access, outcome based on race, region, access to care, socioeconomic status, and other things.
In other words, different demographic groups are affected differently by the health challenges they face and the circumstances in which they face it. Think about the challenges that many people with cancer face and how their specific circumstances impact their care and their experience.
Sherry Davis is a patient the NCI knows who needed cancer treatment in Florida, but couldn’t find a doctor who would take Medicaid that was closer than three counties away. Another patient, Barbara Ingalsbe, drove 100 miles every weekday for radiation treatment.
Several states away, we had Albert Calloway, who had a neck tumor that grew and grew because this individual was uninsured and was overwhelmed by the process of trying to figure out how he fit within the healthcare system. These are three real patients and it’s clear that experiences like this in the United States are entirely too common.
While we’ve made great progress in overall cancer research and care, these benefits have not reached all people equally. The NCI has long sought to address cancer disparities. We were working in this area even before that term, healthcare disparities, really was available in research.
Of course, recent events, and I’m talking about the death of George Floyd, and the disproportionate impact of the pandemic on the poor and disenfranchised. These recent events have injected, and rightfully so I believe, a new focus and passion and commitment to addressing disparities in the entire NIH, including the NCI. That said, these problems are very hard. If their answers were easy, we’d have solved them by now.
Cervical cancer is an interesting example of the complexity of cancer health. So here is a graph showing the incidence of this disease over time, and it shows a very positive trend. There’s been this remarkable decline in cervical cancer incidents in the United States over the last few decades.

We have completely eliminated the difference in incidence between African American and white women. This is good news, and it reflects increased screening for cervical cancer, as well as an effective HPV vaccination. While we should celebrate this progress with regard to this important healthcare disparity, we should also note at the same time that a very large difference in mortality from cervical cancer still exists today.
Even today, Black women in the U.S. are more than 50% more likely to die of this disease than white women. First I think as a scientist, you just have to admit, this is interesting. How can we have so much progress against incidence and not mortality? And why is this cancer so much more lethal in Black women than white women? One can invoke a lot of explanations for this. This could be differences in biology, or differences in risk factors, or differences in access to care, structural racism in the healthcare system, all of these explanations have plausibility.
In cancer health disparities, let me tell you, it’s generally not one of these. It’s going to be a combination of multiple things creating these disparities, but it’s really the business of the National Cancer Institute to figure this out. We should support the research that would identify the causes of these disparities. That’s really the key to fixing these problems.
Race and ethnicity are two features of society that drive healthcare disparities, but there are many other important contributors. Increasingly, we’re appreciating that cancer outcomes are driven by geography, which we think is related to access. For example, we know that people who live in rural communities have worse cancer outcomes, regardless of race or ethnicity.
Cancer incidence and mortality overall are higher in rural areas than in urban ones. This has not always been true in the United States. In the early 1990s, rural patients did better than urban patients, but that trend has reversed and the disparity between urban and rural patients gets worse every year.
This observation holds true for cancer overall, but particularly for cervical cancer, colorectal cancer, kidney cancer, lung cancer, melanoma, and oropharyngeal cancers. Along these lines, a recent study from NCI grantees published this month revealed that women residing in urban areas were significantly more likely to get the recommended colorectal cancer screening compared with women in rural states areas of 11 states.
However, both groups had similar rates of adherence to breast cancer screening, sort of showing how complex this is. You sort of get a different effect of rurality on colorectal cancer screening versus breast cancer screening. That is, colonoscopy versus mammography. But perhaps the most important thing to realize about health disparities research is really the need to stop solely focusing on a single feature of these complex heterogeneous populations. Shown here is a beginning to try and get a handle on this.

This is the topic of persistent poverty, which is defined as 20% of the population living below the poverty threshold for decades. We note that the outcomes of patients living in areas of persistent poverty are worse than patients who are living in areas that are merely currently poor. That is that they’re socioeconomically the same today, but one is that structural poverty going back decades, and that population does worse. Socioeconomic status alone can’t really capture what’s going on here.
We need more sophisticated approaches to understand this interaction between rurality and poverty, particularly through time. We have other examples, for example, the American Indians where overall cancer outcomes are not that bad, but the interaction with poverty in that population is particularly adverse, and we see these terrible pockets of very poor outcomes in the American Indian population, for example.
We have lots of data now showing these nonlinear interactions between things like race and ethnicity and genetics and poverty and rurality, and these interactions can produce some really counterintuitive effects. A key for cancer disparities is to stop single-variable analyses and start working on these populations in their totality, with all their complexity.

As mentioned, the NCI has been interested in the topic of health disparities and minority health for some time. This shows a trend in our funding for these topics dating back to 2010. The NCI has had a significance in this area for over decades, but you can see that has sharply increased in the last few years. Although this is a large investment in this area of science, we believe it is very important to continuously monitor this portfolio, and we think it’s fair to ask if we’re spending on the right topics, or asking the right questions for this field, and spending more in these areas.
We also know the cancer research workforce, the scientists and doctors that do the cancer science, that workforce does not reflect the population of the people we serve. We’ve really redoubled our efforts to make headway against the problem of underrepresentation within the cancer research workforce.
We all share responsibility to change this in whatever way we can and to bake health equity into sort of everything we do. That’s, we believe, an important key to ending cancer as we know it—the president’s goal. Given the lack of diversity in the cancer research workforce, I am excited about several efforts in the NCI to address this problem.
One that is reasonably well known is the NCI’s CURE program. This is the Continuing Umbrella of Research Experiences. This program is a pipeline program that starts in middle school or high school. It provides support for individuals all the way to the junior faculty level. It has thousands of alumni. Some of the most famous researchers in cancer going today are alumni of the CURE program. It really trains them for success. It is the idea that a pipeline is a way to address the lack of representation in science.
Another effort that is different, that is really exciting, is shown here. This is the FIRST initiative. FIRST stands for the Faculty, Institutional Recruitment for Sustainable Transformation. This is a Common Fund initiative, meaning the money to support this comes from the NIH, but is led by the NCI, working in collaboration with National Institute on Minority Health and Health Disparities, NIMHD.

The purpose of the first cohort is to transform the culture at NIH funded extramural institutions, by building a self-reinforcing community of scientists, committed to diversity and inclusive excellence. The rationale here is that a cohort model of faculty hiring sponsorship and mentoring will really sustain support for professional development embedded within an institution that’s committed to workforce diversity.
Here’s the first set of awardees. There are two more rounds of this coming. In fact, the next round of grants is due soon. You see it as a coordinating center at Morehouse and then six awardees. It’s an experiment in this cohort approach, which will include some significant data collection to see if the scientists, the faculty trained through FIRST, will benefit from this program.
You see, the NCI invested in the cohort approach with FIRST, and the pipeline approach through CURE, and we are really trying to consider whatever approach might work best in terms of developing faculty diversity.
Rare and refractory cancers
Let me also talk a little bit about rare and difficult to treat cancers. Just as our advances in cancer research have not benefited all populations, our progress has not been even across all cancer types. You see here, Senator McCain who died of glioblastoma, Ruth Bader Ginsburg, who died of pancreatic cancer, Chadwick Boseman, who died of early onset colorectal cancer.
We are seeing an alarming rise in the rate of colorectal cancer in young patients for reasons that are not clear., The five-year survival rate for glioblastoma, that which affected Senator McCain, is less than 7%, pancreatic cancer is less than 11%. But among these stories, you also see in the upper left corner here, a little girl named Rihanna who had infantile infantile fibrosarcoma, which was heretofore a terrible disease. She was treated with larotrectinib, a TRK inhibitor that allowed her to avoid amputation.
Hers is a success story in a rare cancer that speaks to the long arc of basic science discovery to successful clinical advance. The story of TRK inhibitors for those of you who know it, begins really at the NCI, at Frederick National Lab, back in the 1980s, when Marino Barbacid, working at NCI as a contractor, was hunting for oncogenes, and he found one called onc-D, which turned out to be the first fusion known in cancer involving the TRK gene.
In 2018, larotrectinib, which was used in Rihanna’s cancer, was the first drug approved to treat NTRK gene fusions. It is quite a successful drug for those rare patients that have those events. Another nice example is the DART trial. This is the NCI Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors.

It’s the first immunotherapy trial focused on rare cancers. The DART trial has been tried in many different rare cancers. Here are the results in angiosarcoma, where you can see a patient with a quite bad tumor involving the face and nose, with this very nice response to combine the immuno-oncology approaches.
These results are impressive and encouraging. You can see in about a quarter of the patients, there are these very impressive responses with some patients having their cancers go away entirely. This is remarkable for a number of reasons.
A subtype of angiosarcoma that had been defined earlier through the Count Me In initiative that included about 25% of patients that had high tumor mutational burden and would therefore be a candidate for immuno-oncology. And then this trial happens almost within a year to confirm activity in some patients. The DART is an important platform.
It is not, as I said, solely restricted to angiosarcoma. It is looking at other rare subtypes of cancer, 53 cohorts in all, including cancers of the ovary and intestines and lung and sinuses—rare cancers, wherever they may be found. We think that this is the kind of approach that really has to be taken for these kinds of rare cancers that are not amenable to traditional clinical trials.
The MATCH trial employs this basket approach. When MATCH started, the idea was to sequence patients with refractory cancer and then allocate them to therapy in one of the 40 treatment arms based on the molecular genetics of the tumor. When we started, we thought this might appeal to some patients with rare and uncommon cancers, but in fact, the trial really exceeded our initial expectations with about 60% of those enrolled on MATCH, having cancers other than colon, rectal, breast, non-small cell lung, prostate.
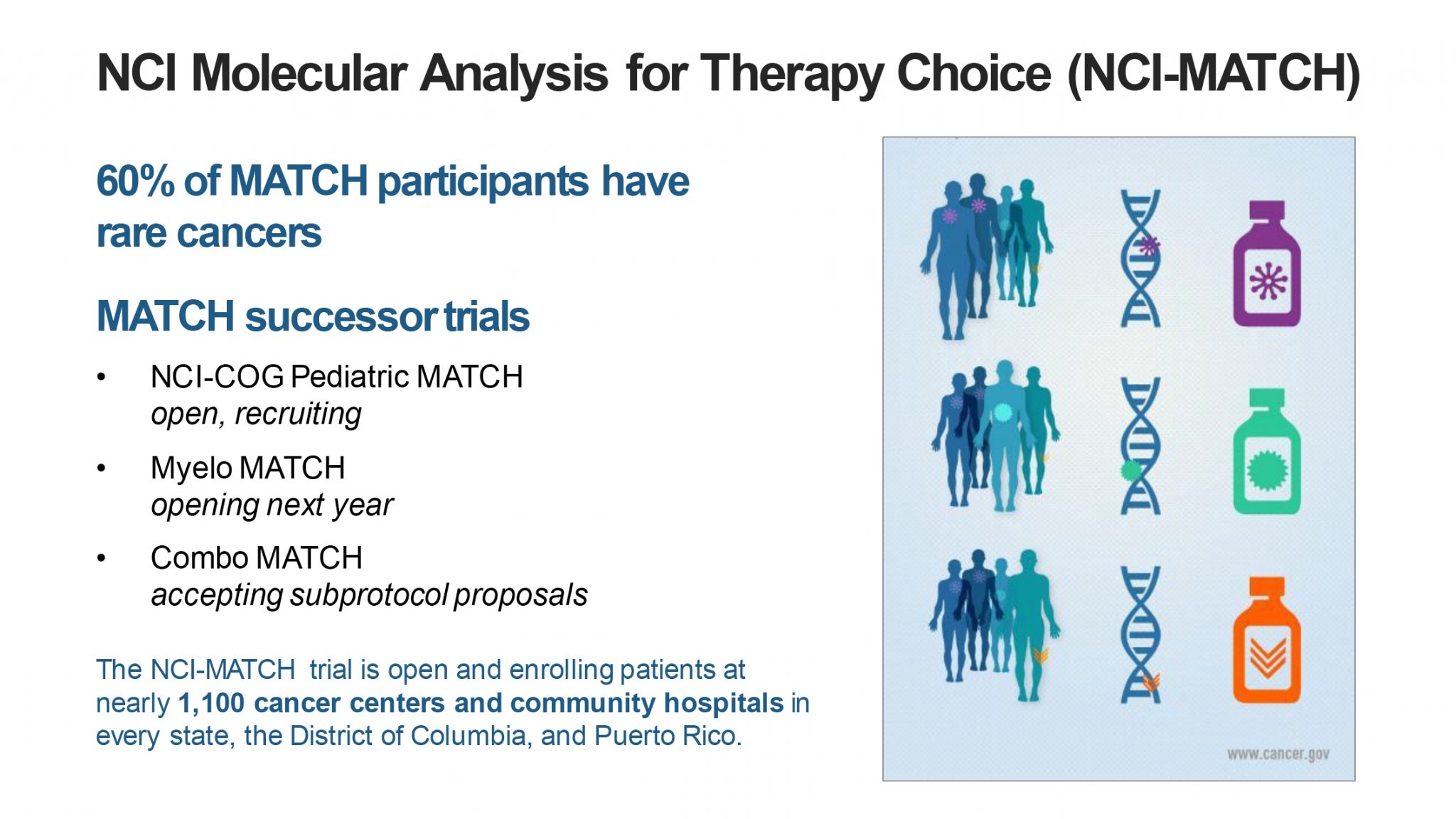
It preferentially enrolled patients from less common cancer types and turned out to be a great rare cancer framework. MATCH, for example, has shown promising results in treating HER2 amplified salivary gland tumors, a rare cancer subtype, treating these patients with T-DM1, producing significant responses in a fraction of the patients.
MATCH is also remarkable as one of the fastest enrolling clinical trials ever done at the NCI. It enrolled patients at 1,100 sites—6,000 patients in just a few years. I think things like MATCH and DART really established this basket trial approach as being quite successful.
Childhood cancer is collectively rare, comprising approximately one to 3% of cancers diagnosed in the United States. This rarity is, as I said, no comfort to anyone who’s watched a child suffer from cancer and its treatment, and it makes our quest to end childhood cancer challenging.
There just isn’t enough data in any sort of one tumor type to really do some of the traditional clinical trials we think of. And so one effort to try and address this problem is the Childhood Cancer Data Initiative. This is a 10-year effort that’s really just begun. It’s in its second year. The idea here is to try to radically aggregate data from children with cancer to make them maximally informative for research and for improved clinical care.

Two important parts of the CCDI are shown here, the Childhood Molecular Characterization Protocol, which would sort of establish a floor of molecular analysis available to every child with cancer United States, and then a National Childhood Cancer Registry, which would try and learn from every trial that would get some data through integration of registry data and various other sorts of datasets that we have to try and get an idea of what happens during the experience of cancers for all children with cancer in the United States. We think these are really important efforts to try and do better in childhood cancer, a collection of rare diseases.
Having discussed some of the challenges we face, cancer as we know it today, the reality is that we will still need more progress for early detection, disparities, and advances in rare and difficult to treat cancers. There are some questions thrown in this slide that are equally important that I haven’t touched on today.
But really, I think they spur us to think about what the future will look like. What are we working toward? If we’re building a bridge to the future of cancer, what’s on the other side of that bridge? A world where these statements are no longer true, where we will have changed cancer as we know it. And I think that future is within our reach. Let’s focus on a future where all people with cancer have the support and resources needed to navigate their care.
Let’s build a reality in which your location, or your race, or your education doesn’t predict the outcome of your disease. And let’s take what we’ve learned and create tests that identify cancer at its earliest stages. And let’s ensure that once these cancers are detected, each cancer can be treated and treated effectively.
This is what the NCI thinks it will take, over this next period. We’ve had this 50 years of progress, now we need to build on that 50 years of progress to advance health equity, to personalize cancer care, to embrace new technologies and innovations, to inspire the next generation of cancer researchers, and to prepare for the challenges of the future.
We know what we don’t know
This is a set of guideposts, the foundations on which we’ll build this bridge to the future. I would argue that we need to look at all our work through a lens of health equity, we need to ask to what extent might this study reinforce existing inequities, or might reflect hidden biases.

You can clearly see how these guideposts are interwoven and overlapping and building the next generation of diverse researchers as part of embracing innovation and creativity.
Today in our age of rapid progress and technical and medical advances, it may be easy to discount the importance of the National Cancer Act. But as 1776 was our nation’s history, and 1969 was the Apollo program that put a human on the moon, 1971 really marks the modern era of cancer research. Maybe this comparison strikes you as a little bit over the top, but I do not believe that is so. Ending cancer as we know it will be as big of a deal for humanity as landing someone on the moon.
1971 is really what got us started. That’s why this anniversary is so important. It was signed into law at a time of great need for those people who feared cancer so much, which at the time was basically everyone. The NCA’s first 50 years was the work of people like Mary Lasker, optimistic politicians, and pioneering oncologists and researchers who were visionary, as I said, but also naïve, as I said.
The optimism induced by the legal mandate and strong infrastructure was soon tempered by the realization that this objective was going to be so challenging. The years ahead will be sharper and focused, different in tone, and more practical, more cognizant of the size and timelines of these challenges, and more based on the foundational molecular biology and biological understanding of cancer.
Over the past five decades, many of us declared, ‘This time is different.’ They weren’t wrong, and that’s what’s brought us so far today. Each time we try this is different. It was reportedly Heraclitus who observed that no one ever steps in the same river twice, the river changes.
In cancer research, we have past thresholds, as compared to 1971. We now have a molecular understanding of these diseases and we’re ready to take a crack at this again. I’ve been trying to make this point for a while now, and I found, actually, a really good analogy that I like a lot, in an excellent book on the history of the National Cancer Act by Abbe Gluck and Charlie Fuchs, both of Yale, entitled A New Deal for Cancer.
It makes a point that I’ve long believed: it points out the optimism for so many of the players held for the rapid cure in 1971—for example, Sidney Farber said he thought a cure for cancer could be achieved by 1976—but as the book notes, the foundational understanding of cancer hadn’t really been grasped in 1971.
There’s this quote from Sol Spiegelman, who was director of Columbia’s Institute for Cancer Research, that I really like, which says, “An all-out effort at this time would be like trying to land a man on the moon without knowing Newton’s laws of gravity.” Fifty years later, we know what we don’t know, and that’s what’s changed. We know how we’re going to end cancer as we know it, when before we really didn’t know that.
Whatever our progress or whatever our successes, it’s certain that they will be possible only because of the work of the last 50 years. And it really built on the work of individuals like Paul Calabresi and the legislation that enabled so much of this work.
I suspect that those who worked so hard to get president Nixon’s signature in 1971 might’ve been disappointed to know that a half century later we’re still losing 600,000 Americans each year to cancer, but I hope they would be gratified to learn that despite the fact that the problems turned out to be so much more complex than we ever imagined, the passion, inspiration, and dedication of the generations that followed have led to astounding progress nonetheless.
Thank you for the opportunity to speak today.
Roy Herbst [Ensign Professor of Medicine and professor of pharmacology; director of the Center for Thoracic Cancers; chief of medical oncology, Yale Cancer Center and Smilow Cancer Hospital; associate cancer center director, translational science]: Thanks Ned. That was wonderful. I think Paul is probably watching from up high and very happy to see all the progress. It’s our tradition at the Calabresi Lecture to ask his brother, Guido, to ask the first question, and I see Guido is on in Italy. Guido, can you hear me?
Guido Calabresi [Paul Calabresi’s brother, Senior Judge of the U.S. Court of Appeals for the Second Circuit, former dean of Yale Law School]: Here I am. I’m in Italy. Can you see me? And can you hear me?
Herbst: We can, this is wonderful.
Guido Calabresi: I was delighted with the lecture because 50 years ago, Paul said to me that the aim was realistically not to end cancer, but to get so that a cancer diagnosis was no different from a diagnosis of high blood pressure or of cardiac problems. So that somebody might live for the longest of time or shortest of time—but that cancer was not a death sentence, but was a life sentence to be dealt with decently and well. And this lecture was so much in that line that it made me smile because that’s what Paul was about.
But there’s something else in this lecture, which really struck me. And that was the continuing difference, even when there are diagnoses at the same time, in results among people because of race, because of poverty, because of all the things that have cursed us in America over so long.
And I just wonder how much—the fact that monies are being given to cancer, as they should, because cancer is such a dramatic disease in people’s mind— how much this can be used, not only to diminish these differences in cancer treatment, but in treatment of diseases, generally? That is, using what is needed to make cancer treatment more equal to different people based on race and poverty, so that all medical treatment becomes more equal in this country. Thank you.
Sharpless: Well, thank you, Judge Calabresi. And it’s a very important question and I think a really important point to make is that addressing the things that drive disparities in health outcomes in the United States will not just benefit patients with cancer.
They would benefit, presumably—we actually have very strong evidence that they would benefit individuals for lots of diseases and would improve health in many ways for the public. So, think about something like tobacco control, which has really been quite successful in certain populations in the United States and not so successful in other populations. Continued use of combustible tobacco correlates with low socioeconomic status and less education. And if we could reach those pockets, the benefits of tobacco control would be way beyond cancer. They would go to many other diseases and general health.
So, it’s a really important question. And we think that—through the last, I’d say 20 years, the NCI’s become very interested in this topic of dissemination and implementation sciences. When you know something works, it works just fine at the tertiary cancer, excellent outstanding academic hospital, but then it doesn’t translate down to the community. What happened there? Why doesn’t that work? And where do things break down?
Obviously many of these things are ascribable to things that we know a lot about—the fractured nature of U.S. healthcare, inequities in education, for example, in the United States.
But, I’m struck by how often disparities are often driven by things that we didn’t appreciate as being so important. For example, a study in disparities in ER-positive breast cancer by race showed that a large part of the disparity was driven by adherence to therapy of the medicine. So, it was really the ability to continue to take the medicine because of the cost of the medicine or presumably the hassle of going to the pharmacy.
I think we had lots of reasons in our mind why that disparity existed, but one of the main drivers was really something so narrow and addressable. So, that’s why I think this line of research is really important. Obviously, the NCI, whether it’s me or on the order of $7 billion a year budget, can’t fix care and education in the United States. Those are much bigger problems, but I think we can do the foundational science that explains what’s really driving these inequities.
Herbst: Thanks Ned. Steven Calabresi has a question.
Steven Calabresi [Paul Calabresi’s son, the Clayton J. and Henry R. Barber Professor of Law at Northwestern, co-founder of Federalist Society]: Thank you, Dr. Sharpless for that presentation. It was really wonderful. There may be an easy answer to this question. But I was curious, given the recent advances in immunotherapy in treating cancer, and given the remarkable success of the mRNA vaccines that Moderna and Pfizer developed against COVID, is there more work to be done on vaccination to prevent cancer? And is that a field that is potentially worth looking into in the future?
Sharpless: Yes. I think that the mRNA platform is very exciting and particularly for the potential, what are called bespoke, totally personalized medicines and certainly an area we’ve been thinking a lot about. Moderna, I think you’re probably aware, started out as a cancer company. Some of their initial products were targeting cancer. And I think Moderna pivoted for a variety of reasons related to technology to vaccines, but still has an interest in cancer and is still supporting clinical trials and cancer patients. And so, I think that this approach makes a lot of sense in the area of personalized vaccines, but maybe other areas as well.
I can tell you that I have one concern about it, that we don’t talk about very much, but I think we should probably talk about more—which is that, having spent time at FDA, the regulatory pathway for bespoke medicines is entirely unclear to me.
It is not certain how you would take medicine that you intend to use in one individual and make that into an FDA approved product under current law. Frankly, I know this is a big turnoff to many of the industry partners in this space who are worried about how they would make a viable product. Even if you could use it in thousands of cancer patients, if the product is different in each patient, a different molecule in each patient, how is that going to work from the regulatory framework?
I think we need some clarity on this topic. I think the FDA needs to provide further guidance on bespoke products and perhaps we even need legislators to write new law in this area. But it’s really exciting, and it goes beyond cancer, by the way. There are many, many rare diseases, particularly rare diseases of children, where these highly personalized medicines can be valuable as well. So, I think as a society it’s really pressing, we figure this out.
Steven Calabresi: Great.
Herbst: Well, listen, we’re at the hour, but we have one special guest. And before I do that, I just want to remind the fellows that we have a half an hour virtual lunch with Dr. Sharpless. So, please stay on this very line. But I’m really excited—Eric Weiner, are you on?
Eric is our new director and actually he gave the Calabresi Lecture about eight, nine years ago. And Eric, I’d just love for you to say a few words, if you have time. I think we missed our window. Well that’s okay. So, Eric was a Calabresi lecturer. We’re very happy that he was here today and he heard your talk, Ned. And I’m sure you have business with him in the not too distant future.
Sharpless: Yeah, I’ve known Eric a while given the Boston connection, and I think what a great development and turn of events to see assume a leadership role at Yale. At the NCI we look very forward to working with him.
Herbst: Great, well, listen, this has been absolutely fantastic. We had one of our largest turnouts for Ground Rounds in the virtual era. What we’re going to do now is I’m going to thank you, Ned. And thank you, Vince. Ned and Vince are going to stay on with me with the fellows. Calabresis, you’re welcome to join us with the group and our trainees, who I’d love to hear a little bit more about these last 50 years, because you’re the ones who are going to take us forward in the next 50 years. So, it’s just been a wonderful day and we’ll look forward to seeing everyone in New Haven in person sometime really soon. So, thank you all very much.
Guido Calabresi: Thank you all. I will go back to picking olives in Italy. Thank you.
Herbst: Bye. We’ll see you soon, Guido. Thank you.


